 |
| Created and Treated Gemstones |
| Flame-Fusion Gemstone Synthesis |
|
The flame fusion process of creating synthetic gemstones was perfected in 1902 by a Frenchman named Auguste Verneuil. Also known as the Verneuil Process, this type of synthesis uses an aluminum oxide powder doped with trace elements to create synthetic corundum of various types. It is also used to make synthetic spinel, although not as much nowadays as it is used for corundum. The process is rather in-depth, but for our purposes I will keep the explanation simple so as to be a bit more understandable for students and consumers. If you wish more study on the Verneuil Process I recommend to visit the Recommended Reading page on www.YourGemologist.com for further study. For now, we will take a look at the process itself to better understand how gemstones are made using this process. The process contains three major components: #1. An aluminum oxide powder which, for ruby synthesis, will have a trace of chromium added. #2. A flame through which this powder is dropped in which it is liquefied. #3. A rotating "boule" which is the formation after the liquid drips through the flame and cools on the rotating seed. Here is an example of the process through graphics: 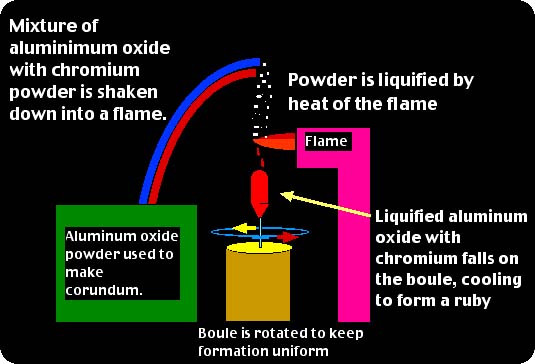
You can see from the boule shown at left that the finished product does not have the crystal shape of a natural ruby. This makes the flame-fusion process rather unique from other synthesis processes in that most of the others will offer a crystal shape in the habit or shape of the natural gemstone. However, with the flame-fusion method, the liquid will crystallize within the same chemical and optical properties, but not offer the same crystal shape. Another interesting aspect of the flame-fusion is that the boules will have a lot of internal stress. This makes for the necessity to split the boules before trying to work with them for faceting and gemstone production. Below you see a side view the split synthetic ruby boule shown at left, and below right you see the boule face on to the split face...showing the structure of the synthetic ruby created by the process.  
Not all synthetics grown by flame fusions need to be split, however, below you see two views of a synthetic blue spinel that is still intact. This specimen has been used a lot on the YourGemologist.com website to demonstrate both the flame-fusion process, as well as demonstrations of the Chelsea filter due to the strong red reaction it gives as a result of the presence of cobalt in its chemical structure.   Identification of Flame-Fusion Synthetics This is one of the easiest of the synthetics to identify based on observation under magnification. And while the Chelsea filter is diagnostic for identifying synthetic blue spinel, neither the Chelsea filter or the spectroscope can be considered diagnostic for synthetic sapphires and ruby. The real identification point for observation under magnification comes through the presence of the curved striations that are almost always present in flame-fusion corundums. These are graphically demonstrated below. And you should get used to looking for these lines whenever you inspect a ruby for possible synthetic origin. Mainly because most of the cheaper synthetic rubies will be made using the flame-fusion method, and these curved lines will be diagnostic in all but a very rare case for synthetics.   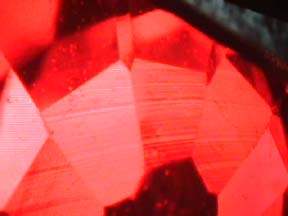 Also present in the flame-fusion synthetics will be gas bubbles...shown below. The photograph at the below left is perhaps the best view you will find on the flame-fusion gas bubbles. And you will most likely see these in synthetic blue sapphires rather than rubies. Although...never say never when it comes to synthetics. But study these images well. There will come a time when you have to see a blue sapphire that you are not sure is natural or not. And these gas bubbles may be all you have to go on.   Other indicators There are other indicators of synthetic corundum by flame-fusion...but you should not attempt to make a call for syn -v- natural using these methods until you are well experiences with known stones. The first is fluorescence. Due to the absence of iron in most synthetic rubies, they tend to react much brighter to UV light than naturals. Again, this is only an indicator and not diagnostic. But once you see enough syn. rubies, you will start to see the indicator of UV. The final is the spectroscope. There is so much chromium put into a synthetic flame-fusion ruby, that the chromium lines literally jump out at you. But again, you have to be experienced enough looking at chromium lines to know what you are looking at. So until you have looked at enough synthetic and natural rubies to know the difference, don't try to make this call on your own. Look for help from others more experienced. The bottom line is, you are going to see a lot of flame-fusion synthetics out there. And they can range from rubies to sapphires to spinels and even to a vanadium doped corundum that emulates alexandrite. If you look for the curved striations, look for the gas bubbles, and the overall general perfection of the gemstones that just seems too good to be true....those will give you a good indicator of a flame-fusion synthetic gemstone. |
Back to Created and Treated Study PageBack to Home Page |
| © Copyright 2014 All Rights Reserved. Please read the fine print below:The information contained in this website is offered free of charge to anyone wishing to learn more about gemology. The information may be downloaded by any student, consumer, or jeweler for your own personal study and use. None of this site can be downloaded for posting on another website or server for any reason. It will be a violation of the copyright for anyone to copy, duplicate, distribute, and/or re-print this material in any format or any medium without written permission. Nor can anyone post this information on a for-profit website without written permission. That will ruin it for everyone and cause the entire site to be erased and canceled. Please honor this copyright for the good of everyone else. |
 Introduction.
Introduction.